High Hydrostatic Pressure stands as a promising option for sanitizing materials made using AM.
Additive manufacturing (AM) has recently gained popularity in several medical fields due to the potential to construct prototypes and biomedical devices (Gao & Cui, 2016). Employing AM with biomaterials is promising for developing customized patient-oriented applications, such as implant fabrication (Bose et al., 2017) and the development of human organs (Radenkovic et al., 2016). While there is a current trend in replacing metals, alloys, and ceramics for polymeric materials, there are special considerations that medical devices should fulfill to perform their function of minimizing the adverse effects of a toxic or immunological response due to the interaction of tissue with the device. Polymers are extensively employed due to their superior attributes, such as their supporting robustness, extended use capacity, and facility to meet specific requirements, such as adhesion or drug release (Bernard et al., 2018).
In general, sterilizing devices or elements of biomedical prototypes produced with AM have been neglected. However, implants and other long-term or life-supporting apparatus require treatment before encountering human tissue. Compatibility among the manufacturing methodology, the material, and the sterilization method should be assured to comply with both the biocompatibility requirements and the highly regulated environment. Moreover, regarding the disinfection and sterilization of medical equipment (patient-care items or equipment), the type of sanitization has been divided into three categories-namely, critical, semi-critical, and noncritical-on the basis of the degree of risk of infection involved in the use of the items (Spaulding, 1968). The taxonomy comprises items of a broad spectrum of conditions; critical items are those associated with infection if the item is contaminated with any microorganism or spore; semi-critical items are those in contact with mucous membranes or nonintact skin, such as endoscopes, anesthesia equipment, or esophageal manometry probes; and noncritical items are those that come in contact with skin, but not mucous membranes (i.e., blood-pressure cuffs, crutches, bed rails, or linens) (Rutala & Weber, 2004). This taxonomy has been adopted and promoted by the Centers for Disease Control and Prevention (Sehulster et al., 2003). Due to the dissimilar and ubiquitous reach of additive manufacturing, research devices could be considered in any of the mentioned categories.
Most studies on the implementation of implants based on additive manufacturing have focused on the effects of the parameters of design, materials, or process. However, personalized additive manufacturing has to deal with the mechanical properties and biocompatibility of the printed material after the sterilization method (Jamróz et al., 2018). Nonetheless, current state-of-the-art regulations are centered on non-polymeric orthopedic-based materials. The Food and Drug Administration of the United States (FDA) has established dry heat, ethylene oxide, steam, and radiation for orthopedic implants (Class III implants) («FDA Classify Your Medical Device,» n.d.; FDA, 2016). Although other sterilization techniques (i.e., hydrogen peroxide and ozone) are under review, the agency has not approved these methods. New sterilization methods, such as pulse light, microwave radiation, and vaporized peracetic acid, have also been reported (Tipnis & Burgess, 2018).

Additive manufacturing technologies have shown significant potential for producing biomedical devices (Singh et al., 2017). For example, Point-of-Care (PoC) solutions have shown the potential to be implemented as ultra-low-cost solutions (Martínez-López et al., 2016; Martínez-López et al., 2017). Regardless of the cost-effectiveness ratio, the capacity to produce complex structures with polymers is challenged by the mechanical properties of the products and by the limitations that arise from the availability of materials to be processed (Ngo et al., 2018).
HHP, as a sterilization method for 3D-printed parts, has not been reported for biomedical engineering, even though it has been widely used for food preservation (Yordanov & Angelova, 2010). The requirements of an increasing market of health-conscious consumers for high-quality edible products that maintain freshness, nutritional value, and flavor while decreasing the number of additives employed have boosted the development of non-thermal preservation technologies, such as high pressure, a pulsed electric field, pulsed light, an electron beam, plasma, and modified atmosphere packaging. Hydrostatic high-pressure (HHP) sterilization does not rely on heat, chemicals, reduced water activity, or reduced temperatures to control pathogens or microbes (Sugita et al., 2014). Cellular processes are impaired under high pressure; motility, cell division, and protein synthesis are turned off under 10, 20, and 50 MPa, respectively (ABE, 2007). High pressure unfolds proteins, causing the protection provided by the wall membranes to be disrupted until viability is compromised (Farkas, 2016). This technique consists on the appliance of intense pressure (100–1000 MPa) to cause microbial inactivation (Yordanov & Angelova, 2010). HHP has been used extensively to process fruits, vegetables, meat, and dairy products.
An HHP system comprises a vessel and its closure pressure-generation system, temperature control device, and material-handling system. Pressure-transmitting fluids are used in the vessel to transmit pressure uniformly and instantaneously. The samples should be packaged in flexible packaging and loaded in the vessel. A pump or piston is activated to apply the pressure energy and maintained for the desired time; then, the vessel is decompressed. Depending on the application, the compression, holding, de-compression, loading, and unloading can be repeated as required for the application (Castro & Saraiva, 2014). A recent review of the state of the HHP industry estimated that every year, 500 thousand of tons of products are processed by around 300 industrial machines in production (Huang et al., 2017).
For this paper, we propose high-hydrostatic pressure as a sterilization method to increase the biocompatibility of biomedical device materials. The design and manufacturing of patient-specific devices is a task of growing interest among the medical community. For example, intervertebral implants have received much attention in consideration of the potential benefits to patients derived from the customization of an implant. Figure 1 illustrates the application of the proposed technology for fabricating an intervertebral implant that could be applied to other medical devices manufactured using additive manufacturing technologies.
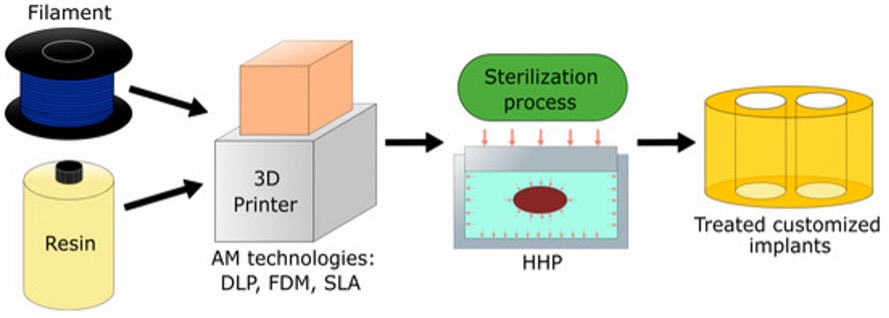
Figure 1. The schematic process for fabrication of medical devices (e.g., implants) using AM technologies.
In a recent review, Tipniss and Burgess acknowledged the high sensibility of polymers to the sterilization techniques for polymers, either as a protective coating or while being the substrate of medical devices (Tipnis & Burgess, 2018). Sterilization is defined as a process in which living cells, viable spores, viri, and viroids are eradicated or removed from an object. Autoclave (or steam sterilization method) has been considered as a nontoxic, easy to control and monitor, rapid sterilization technology that is available in virtually any medical facility; however, the methodology can be deleterious to heat-sensitive instruments and instruments can be damaged by exposure or rust originating from humidity (Rutala & Weber, 2004).
The proliferation of additive manufacturing (AM) equipment with a higher resolution has shifted the aim to develop visual prototyping models to end-user parts. Personalized medicine based on tomographic images and the capability to produce pieces with a high complexity have driven the development of a growing interest in additive manufacturing for producing orthopedic implants. However, limitations on the choice of materials, such as the porosity of the parts due to the underlying manufacturing process, mean that there are numerous prototypes of devices with inferior mechanical properties than those fabricated using subtractive or formative technologies (Ligon et al., 2017).
There is an inherent demand for high mechanical properties and biocompatibility of AM implants and other medical devices. Espalin et al. (Bourell et al., 2010) proposed the use of Fused Deposition Modeling (FDM) with a medical-grade PMMA filament and found that porosity and fabrication conditions influence the mechanical properties. Domanski et al. (Domanski et al., 2015) proved that Selective Laser Melting (SLM) can produce parts with higher mechanical properties compared to Fused Deposition Modeling (FDM) and Powder Based 3D Printing (3DP).
Additional to the natural effect of AM as a process, recent research suggests that sterilization procedures may affect the structure, aggregates, and properties of AM materials (Puppi et al., 2012).
Flege et al. (Flege et al., 2013) studied the effects of different target doses and irradiation of γ-sterilization on the molecular weight of SLM-manufactured materials, but the mechanical properties were not addressed in their evaluation. Béduer et al. (Béduer et al., 2018) proposed an AM technology that is viable for 3D printing scaffolds used in tissue engineering and considered an autoclave sterilization method after printing. However, the effects of the sterilization were not quantified. Obaton et al. (Obaton et al., 2017) also considered autoclave sterilization after printing an SLM-processed part, but the effects were not measured.

Following a different approach, Neches et al. (Neches et al., 2016) proved that it is possible to obtain 3D-printed products that are sterilized during the curing process of the material, meaning that no further sterilization procedure is necessary. Furthermore, Zuniga (Zuniga, 2018) reported the use of FDM for 3D printing sterilized prostheses by using an antibacterial polymer filament.
Studying the effects on the mechanical properties of AM materials due to sterilization techniques could provide insights for improved biocompatibility. This work presents the effects of the mechanical properties of high-hydrostatic pressure (HHP) processing as a sterilization method on samples manufactured with different AM technologies. Three different AM methods are subject to assessment and compared with a standardized autoclave methodology. To evaluate the feasibility of the proposed methodology, we have employed commercially available equipment and reagents. While it would be interesting to examine the suitability of the novel methodology to process different types of risk and devices, and hence the potential to comply with medical policies, we have purposely excluded this discussion.
Do you want to know more about our study? Click Here!


